SOME MONOCLINIC
MINERALS

BRAZILIANITE

AEGIRINE
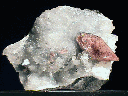
AZURITE

BORAX

CATAPLEIITE

MUSCOVITE

HUEBNERITE

CROCOITE
|  |
The monoclinic system is the largest symmetry system with almost a third of all minerals belonging to one of its three classes.
This system contains two non-equal axes (a and b) that are perpendicular to each other and a third axis (c)
that is inclined with respect to the a axis. The a and c axes lie in a plane. The a-c plane can be, but
is not always, a mirror plane with the left side of the b axes a reflection of the right side. When viewed down the b
axis, it becomes apparent that the b axis can be, but is not always, a two fold rotational axis. It can turn the crossed, but
not perpendicular, a and c axes 180 degrees and repeat the identical structure; just like an X can be turned upside
down and still be the same X. When the two symmetry operations are present together, the mirror plane is perpendicular to the two
fold rotational axis and the symbology, 2/m (expressed 2 over m), is used. If you followed the above discussion, then you have
a good handle on the three classes possible in the monoclinic system. The lowest symmetry class has a mirror plane and lacks the
two fold axis. The other low symmetry class has the two fold rotational axis but lacks the mirror plane. Which is lower in
symmetry depends on the outcome of the argument: is a mirror a higher symmetry operation than a two fold rotation?
The highest monoclinic symmetry class is the 2/m class with both operations being present. The 2/m class is a very
popular class as more minerals form a structure with this symmetry than any other. By comparison, the other two classes
of the monoclinic system are found lacking with a significant decrease in minerals representing them.
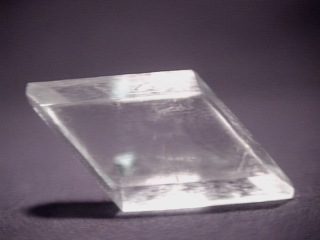
GYPSUM
- Class: 5th
- Symmetry: 2/m
- Symmetry Elements: One two fold axis with a perpendicular mirror plane
- Axes: None are equal
- Angles: Alpha and beta angles = 90 degrees but gamma does not.
- Common Forms: The monoclinic prism and the pinacoid.
- Most Common Minerals Known to this Class: This is the largest symmetry class in terms of the number of minerals with almost
eleven hundred recognized members. Some of the more noteworthy members are
Acanthite,
actinolite,
aegirine,
allanite,
annabergite,
arfvedsonite,
arsenopyrite,
augite,
aurichalcite,
azurite,
bannisterite,
beryllonite,
biotite,
borax,
boulangerite,
brazilianite,
brochantite,
butlerite,
calaverite,
carnotite,
catapleiite,
caledonite,
celsian,
chalcocite,
charoite,
chondrodite,
chrysotile serpentine,
clinochlore,
clinoclase,
clinoptilolite,
colemanite,
cookeite,
cornwallite,
creedite,
crocoite,
cryolite,
cryptomelane,
cummingtonite,
datolite,
diopside,
dufrenite,
edenite,
epidote,
erythrite,
esperite,
euclase,
fluorrichterite,
gadolinite,
gaylussite,
gibbsite,
glauberite,
glauconite,
graftonite,
gypsum,
harmotome,
hedenbergite,
hessite,
heulandite,
hodgkinsonite,
hornblende,
howlite,
hubnerite,
hydroboracite,
hydromagnesite,
hydrozincite,
illite,
jadeite,
jamesonite,
jordanite,
kernite,
kidwellite,
kieserite,
kinoite,
kottingite,
kovdorskite,
ktenasite,
lamprophyllite,
lanarkite,
langbanite,
laumontite,
lazulite,
leadhillite,
legrandite,
leucosphenite,
linarite,
liroconite,
ludlamite,
malachite,
manganite,
melanterite,
monazite,
montmorillonite,
muscovite,
olivenite,
orpiment,
orthoclase,
palygorskite,
papagoite,
pargasite,
phillipsite,
phlogopite,
polybasite,
polylithionite,
pseudomalachite,
psilomelane,
pyrrhotite,
realgar,
richterite,
riebeckite,
romanechite,
rosasite,
roselite,
sanidine,
sauconite,
semseyite,
sklodowskite,
sphene,
spodumene,
staurolite,
stilbite,
stringhamite,
sussexite,
sylvanite,
synchysite,
tainiolite,
talc,
tenorite,
thomsenolite,
titanite,
tremolite,
trona,
vermiculite,
veszelyite,
vivianite,
volborthite,
whewellite,
whiteite,
wohlerite,
wolframite,
xonotlite,
zinnwaldite,
zippeite
and zirconolite-2M.
^

MESOLITE
- Class: 4th
- Symmetry: 2
- Symmetry Elements: One two fold axis of rotation
- Axes: None are equal
- Angles: Alpha and beta angles = 90 degrees but gamma does not.
- Of Note: Crystals can be left or right handed, enantiomorphic, and hemimorphic, having different tops and bottoms.
- Common Forms: The sphenoid,
pinacoid and pedion.
- Most Common Minerals Known to this Class: Amicite,
boltwoodite,
franklinfurnaceite,
goosecreekite,
halotrichite,
joaquinite-(Ce),
mesolite,
miargyrite,
pickeringite,
remondite-(Ce),
rinkite,
uranophane,
wollastonite-2M
and many organic chemicals crystallize in this class including sugar and tartaric acid.
There are roughly 70 minerals belonging to this class.
^

NEPTUNITE
- Class: 3rd
- Symmetry: m
- Symmetry Elements:
One mirror plane of symmetry
- Axial Lengths: None are equal
- Angles: Alpha and beta angles = 90 degrees but gamma does not.
- Common Forms: The dome,
pinacoid and pedion.
- Most Common Minerals Known to this Class:Alamosite,
antigorite (serpentine),
clinohedrite,
natron,
neptunite and
scolecite
Roughly 40 minerals belong to this symmetry class.
^
Isometric
| Tetragonal
| Hexagonal
| Trigonal
| Orthorhombic
| Triclinic
| Amorphous
|